Microscopes: Looking deep into hidden worlds
Many people are as fascinated by the microworld and the nanoworld as others are by the cosmos or the deep seas. These worlds seem to be inaccessible, apparently hidden from human eyes. However, across a wide range of disciplines, microscopes make it possible to take ever deeper and more precise looks at the smallest of details, and in ever higher resolutions – right down to atomic structures. The equipment which researchers use for this is highly complex and has almost nothing in common with the simple optical microscopes which many of us know from our schooldays. Fluorescence microscopy, for example, is a method which uses optical microscopy to label biomolecules within the specimen with fluorescent dyes and make the light they radiate visible. In research work, a number of other microscopy methods are used which are based on other physical principles such as electron and scanning probe microscopy. On this theme page we look at some of the equipment used by researchers working in the fields of natural and life sciences at the University of Münster, taking a look at their research.
Low-temperaure scanning tunnelling microscope
Just one single atom which is missing or in some way different can influence the properties of ultra-thin, two-dimensional materials. For example, such a defect can lead to the material emitting individual photons – and that makes it interesting for possible opto-electronic applications. It is mechanisms such as this that the working group headed by Prof. Anika Schlenhoff at the Institute pf Physics is interested in. How, for example, do the structural properties of 2D materials relate to their electronic or magnetic properties? The team examines the surfaces of samples using a scanning tunnelling microscope, which can make every single atom on the sample’s surface visible – as well as the gaps, if an atom should be missing. “The smaller the dimensions of the 2D material, the more significant are atomic defects,” explains Anika Schlenhoff. “When we understand for example how such flaws change the properties of a material, we can generate them with the specific aim of controlling the properties,” she adds. The scanning tunnelling microscope belongs to the group of scanning probe microscopes, with which a measuring probe scans the surface. A fine, conductive measuring tip glides just above the electrically conductive surface, without actually touching it. The so-called tunnel current which flows between the probe and the tip allows conclusions to be drawn regarding not only the distribution of electrons but also, as a result, the structure of the surface – as far down as the arrangement of individual atoms and how they differ.
The device which the Schlenhoff team uses is housed in the Center for Soft Nanoscience (SoN) and works at very cold temperatures down to below five Kelvin (approx. -268 degrees Celsius). These cold temperatures ensure a stable positioning of the measuring tip over the individual surface atoms, as well as providing a high quality of measuring signal. A special feature which the device has is the fact that it is linked up to a test set-up which allows an optical stimulation of the surface of the sample. This enables the team to make conclusions about the opto-electronic properties of the sample and relate them to the structural and electronic properties.
Author: Christina Hoppenbrock
Infrared microscope with spectrometer
For two years now, the working group headed by Prof. Harald Hiesinger at the Institute of Planetology (Faculty of Geosciences) has had at its disposal an instrument of which there are only a few in this combination in Germany: a spectrometer with a built-on infrared microscope – costing around 350,000 euros. The important thing about spectroscopy is that different materials – in this case minerals – absorb, reflect or emit certain light. This produces a spectrum which is characteristic for the material in question. However, it is difficult to define the spectrum of pure minerals as these mostly display impurities in nature which change the spectrum. This is where the microscope comes into play. It makes it possible to examine individual points on a sample which are sometimes only a few micrometres in size. The microscope works in the medium infrared spectrum with light having wavelengths of between two and 20 micrometres. The point on the sample which is magnified by the microscope is radiated with infrared light in order to then enable the reflected light to be examined in the spectrometer.
The aim is to build up a database in which the spectra of as many materials as possible are stored, enabling different minerals with their own “fingerprint” to be identified later. This fundamental understanding of spectra helps for example in examining the surface and composition of various celestial bodies. The BepiColombo space probe is currently heading towards Mercury and has a spectrometer on board which is being monitored by Harald Hiesinger’s team. When the data provided by the probe are available, beginning in 2026, it will be possible to evaluate them thanks to the spectrum database.
Author: Patrick Dietz
High-performance cryogenic electron microscope
The working group “KryoEM komplexer Nanosysteme” (CryoEM of Complex Nanosystems) at the Institute of Medical Physics and Biophysics at the Faculty of Medicine devotes itself to explaining the architecture of complex nanomachines by visualizing them directly by means of cryogenic electron microscopy. This often involves examining target proteins inside cells. In addition to carrying out its own research, the team also provides support for researchers from the natural and life sciences at Münster University who want to investigate the finest details of proteins. The working group, which is headed by biologist Prof. Christos Gatsogiannis, has a high-performance cryogenic electron microscope (cryo-EM) at the Center for Soft Nanoscience which is second to none. Overall in Germany, there are only a handful of comparable devices.
The cryo-EM can visualise the most minute components of a cell down to individual atoms. This means that the researchers can trace the processes in the interior of a cell. The microscope is also able to visualise three-dimensional protein structures. The cryo-EM is a transmission electron microscope, which depicts microscopically small objects through electron radiation at extremely low (cryogenic) temperatures. “In order to speed up the electrons, we need voltage of 300,000 volts,” says cryo-EM expert Dr. Alexander Neuhaus. The high-performance cryo-EM is capable of a resolution of almost one angstrom, which is about one ten-millionth of a millimetre – corresponding to the dimension of atomic radii. The device’s digital camera and processing technology can produce up to 20,000 individual images in 24 hours. Each image file is 16 megabytes in size; the data are processed using the University’s own PALMA II high-performance computer. Around 15 working groups are already using the infrastructure, which cost 7.5 million euros and was purchased with financial support from the German Research Foundation and the state of North Rhine-Westphalia. It was inaugurated in spring 2023. Further groups are set to follow.
Author: Christina Hoppenbrock
Three-photon microscope
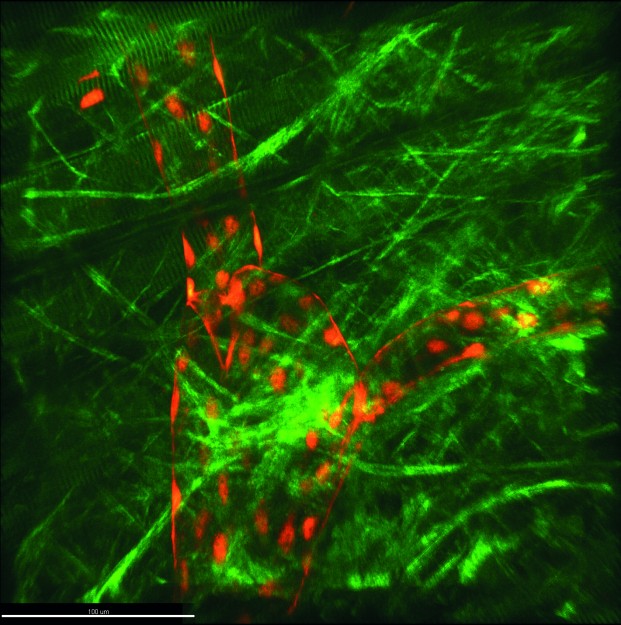
For some weeks now, there has been a new piece of technical equipment in the “Intravital Molecular Imaging” working group headed by Prof. Friedemann Kiefer (Faculty of Medicine). This device, which makes many a researcher’s heart beat faster, is the so-called three-photon microscope, and it was built especially for these researchers and their requirements. “Worldwide, there are only a few devices of this calibre,” says Friedemann Kiefer. “Light scattering and absorption are factors which limit the imaging depth attainable in optical 3D microscopy, and this device enables us to reduce these more than we could in the past. We can now create three-dimensional images of living tissue which are not possible in imaging elsewhere.” Three-photon microscopy is a method of fluorescence microscopy. Researchers use this technique in order to examine processes in living organisms down to the molecular level – for example, oxygen supply or the reaction of organs to stimuli such as inflammations.
Microscopy carried out on a living organism is known as intravital microscopy. Instead of using energy-rich short-wave excitation light – as in confocal microscopy – three-photon microscopy uses particularly low-energy long-wave light in order to excite a fluorescence label. The long-wave infrared light scatters less and can penetrate more deeply into living tissue. As a result, three-photon microscopy makes imaging possible up to one millimetre in the sample – and sometimes deeper – with very slight background signals. As this method requires three photons to interact simultaneously with a fluorescence label, very short laser pulses are used, lasting just a few femtoseconds and with a high photon density. One positive side-effect of the extremely short pulse duration is the minimal damage done to the living tissue.
Author: Kathrin Kottke
Confocal laser scanning microscope
Cellular processes in the development of organs are the focus of researchers in the working group headed by Prof. Stefan Luschnig at the Institute of Integrative Cell Biology and Physiology in the Faculty of Biology – for example, the remodelling of cell contacts in the fruit fly. In order to visualise these processes down to the level of molecular detail, they use a confocal laser scanning microscope – a special fluorescence microscope which depicts a detailed picture of the structures in cells at a resolution of 200 nanometres. In the process, an optical trick is used: while normal optical microscopy illuminates the entire sample, the confocal microscope uses a laser which scans the area to be examined point by point. Software is subsequently used to piece the information together to create an image – for example, the ovaries of the fruit fly in which certain proteins have been labelled genetically with fluorescent molecules. In creating the microscopy image, the fluorescent light is concentrated together through a pinhole aperture and the areas outside the focus are masked out. In this way, an image is created without any unwanted overlays. “This means that we can create extremely sharp images of the inside of embryos and cells,” says Stefan Luschnig. “Just how well researchers can now see inside cells was barely conceivable a few years ago. Now we can observe cellular processes as they happen ‘live’, so to speak.”
Since 2018 the device, which cost 400,000 euros, has been available not only for Luschnig’s team to use, but also for all researchers at Münster University. The microscope, and many other pieces of equipment besides, can be booked for use via the ‘Imaging Network’, a collaborative network at the Cells in Motion Interfaculty Centre.
Author: Kathrin Kottke
Background: research infrastructure available for use by everyone
The confocal laser scanning microscope which the Luschnig team has, and the three-photon microscope that the Kiefer team uses, are part of the ‘Imaging Network’ – a collaborative network at the Cells in Motion Interfaculty Centre which comprises 50 pieces of equipment. Via the network, all researchers at the University of Münster can use equipment, methods and expertise and also place these at the disposal of others. There are currently over 500 people using the network.
This article is from the university newspaper wissen|leben No. 6, 4 October 2023.